
STUDY BY: Dr Donald Murphy 1,2, Dr Brian Meade 3, Dr Avni Sali 3. LOCATIONS: Geelong Private, St John of God & Colac Hospitals 1, Skills Laboratory RACS. Melbourne2, National Institute of Integrative Medicine Melbourne3.
Introduction
This phase 1 ethics approved trial has been established to assess the potential role of Sono and Photo-dynamic Therapy using Radachlorin, Sonnelux and Photosoft sensitisers in a cohort of 66 patients, across a range of biopsy-proven prostate cancer patients. Including patients with focal disease who are under surveillance and also patients with persisting cancer, after radical treatments.
History of Light Therapy
1903 Nobel Prize N. Finsen – Light therapy. 1904 Von Tappeiner – PD reaction first shown. 1925 Nobel Prize H. Fischer – Porphyrins. 1942 Porphrins – First used in treatments 1958 Light Bed therapy for Neonatal Jaundice. 1971-85 Dougherty – Photofrin therapy. Russian and Chinese PDT developments Western interest in PDT applications. 1990 Windahl et al. – PDT for local prostate cancer. 2006 Moore & Emberton. – Interstitial PDT.
Aim
To investigate the role of sono and photo-dynamic therapy in the treatment of prostate cancer.
Method
The sensitiser is taken sub-lingual or orally 16-24 hours before each treatment cycle. The laser and ultrasound probes provide the energies which are directed at the prostate in a combination of trans-rectal, trans-urethral and per-cutaneous techniques. Energy delivered AM and PM each day.(25 min max.) for 3 days over one week and then repeated twice in 12 weeks. Laser red light at a maximum of 2 watt at 4000-5500 Joules. Ultrasound set to a maximum of 1 watt and at low frequency. The treatment is performed as an Outpatient procedure with eye protection provided.
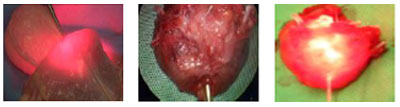
Picture 1: Experimental proof of trans-rectal illumination in a pig model. Picture 2 & 3: Prostate speciment with & without illumination.
Results
For 26/40 patients now treated for > 6 months. The dosage of the sensitisers as well as the spectral range of the two energies used, have been shown to be safe. There have been no safety issues. The sensitisers used have displayed no systemic toxicity or sun sensitivity. The interim results are as follows:- 1. The flow studies, IPSS profile and Quality of Life due to urinary symptoms, recorded low scores as improved or unchanged except for one patient. 2. The PSA patient data for > 6 months post treatment :- (a). stable or decreasing in 13/26. (b). Increasing in 8/13 (neg. Bone/CT scans) with a positive prostate biopsy and 3/13 a negative biopsy. (c). Increasing PSA (positive lymph nodes on Abd. CT) in 2/13. 3. UTI In 2 patients, cleared on treatment. 4. No urinary incontinence or rectal injuries. 5. One 79 year old patient with increasing obstructive urinary symptoms was treated by TURP. 6. Decreasing prostate size on assessment. (DRE, Ultrasound & MRI) 7. No alteration in erectile function.
Conclusions
The phase 1 criteria have been met regarding the sensitiser doses and equipment safety. Normal bladder and bowel function, as well as unaltered potency have been recorded. A stable or decreasing PSA has been recorded in 13/26 patients . An apparent / proven decrease in prostate size (ref.1) has been noted. This experimental treatment may have a future in the management of prostate disease.
References
1.Moore, C. M., Emberton, M. and Bown, S. G. (2011), Photodynamic therapy for prostate cancer-an emerging approach for organ-confined disease. Lasers in Surgery and Medicine, 43: 768-775. doi: 10.1002/lsm.21104
Acknowledgements
I wish to record my gratitude to the Companies supplying the agents and also the relevant equipment. I also record my thanks to Paul Fargher at Geelong Physiotherapy for providing his Ultra-sound equipment. There has been no financial payment by the companies for this study.
